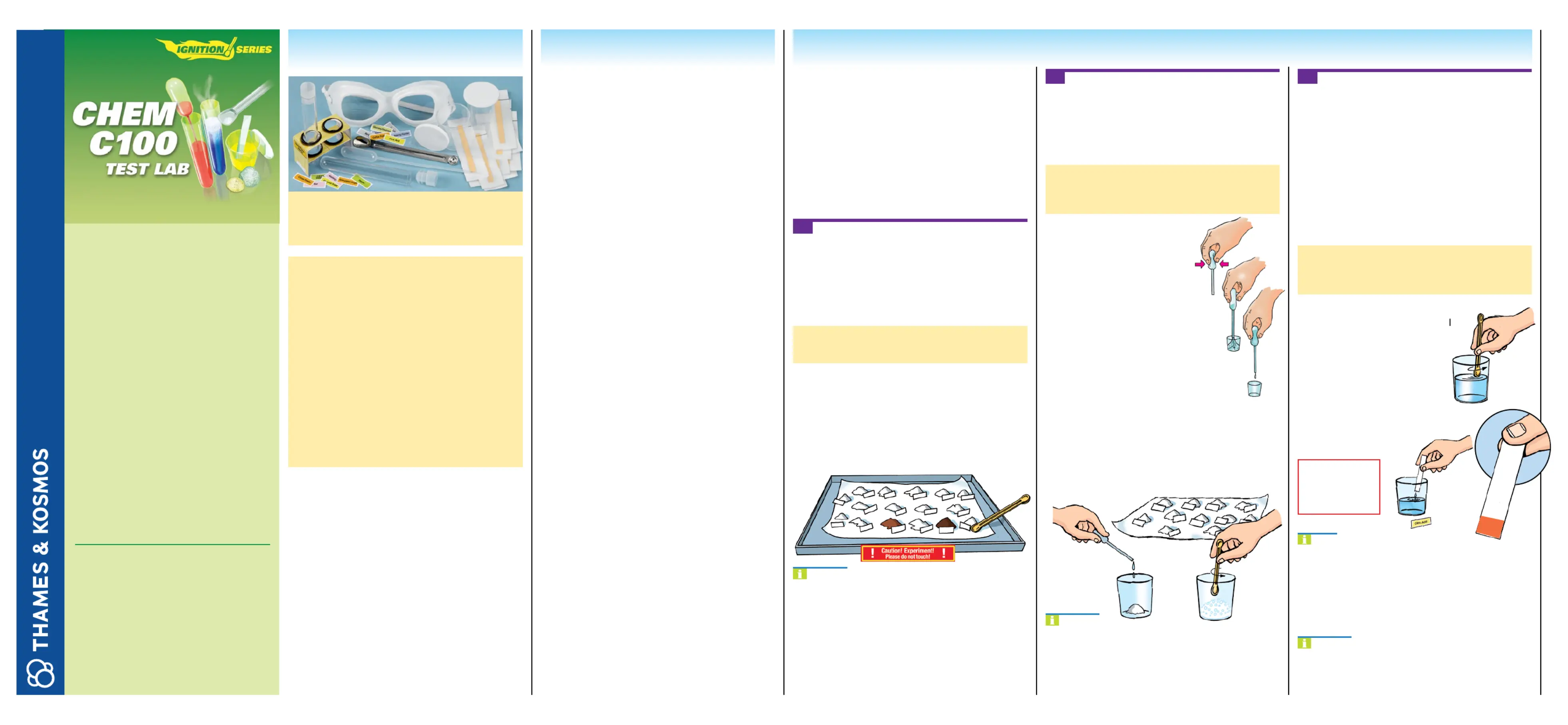
Thames & Kosmos CHEM C100 Test Lab Handleiding
Thames & Kosmos
Niet gecategoriseerd
CHEM C100 Test Lab
Bekijk gratis de handleiding van Thames & Kosmos CHEM C100 Test Lab (2 pagina’s), behorend tot de categorie Niet gecategoriseerd. Deze gids werd als nuttig beoordeeld door 4 mensen en kreeg gemiddeld 4.5 sterren uit 2.5 reviews. Heb je een vraag over Thames & Kosmos CHEM C100 Test Lab of wil je andere gebruikers van dit product iets vragen? Stel een vraag
Pagina 1/2
This experiment kit is intended for children over 8
years of age. Select the experiments that you think
are appropriate for your child. Before starting,
please read through these instructions, the safety
rules, and the first aid information, follow them,
and keep them on hand for reference. The incorrect
use of chemicals can lead to injury or other health
risks. Only carry out experiments that are described
in the instruction manual. The area around your
work place should be kept free of all obstructions,
and it should be sufficiently far from food storage
areas. It should be well lit and well ventilated, and
equipped with a water tap. There should be a solid
table with a rugged, fire-resistant surface that you
can wipe off. During all experiments, the safety
glasses should be worn to protect the eyes.
Rule for Safe Experimentation
1. Read the experiment manual before starting the
experiments, follow its instructions and keep it on hand
for ready reference.
2. Thoroughly prepare your work area. Clear off the table
and make sure that all the things you will need are ready.
3. Only perform the experiments described in this manual.
If safety precautions are mentioned, be sure to follow
them.
4. Always wear the safety glasses when performing the
experiments. If something gets into your eye by mistake,
such as a squirt of citric acid solution, rinse your eye
thoroughly with water. Let an adult help you.
5. When you are done, cleaned all the equipment that you
used and always leave your work area clean. Any leftover
solid substances can be thrown into the garbage, and
liquids can be rinsed down the drain with plenty of water.
6. Any investigated foods must be disposed of afterwards.
7. Do not eat or drink while performing experiments.
8. Provide necessary fire protection when experimenting
with candles. Set the candle on a fireproof base. Never
leave burning candles unattended, and extinguish them
after the experiment.
9. If you spill anything, wipe it up immediately with a
paper towel.
10. When performing experiments, wear old clothes that
you don’t mind getting dirty.
11. Wash your hands thoroughly after completing your
experiments.
General First Aid Information
In case of contact with eyes and in case of injury: Rinse the
affected area with plenty of water and in case of injury
always seek medical help. In case of swallowing: Rinse
mouth with water and drink fresh water. Do not induce
vomiting. Seek medical help without delay. In case of
inhalation of dust: Bring the individual into fresh air.
01
Experiment
Using Your Eyes and Nose
As a chemistry detective, you should try to get
to know as many different substances as possible
from your environment — particularly the common
chemicals found around the house.
You will need: measuring spoon, 16 small name
cards, paper, 16 test substances from the Addi-
tional Items section on the Contents panel
Take one sample of each substance with the mea-
suring spoon and place the samples on a piece of
paper. You will have to crush up the glucose tablet
into a powder. Ideally, lay your sheet of samples on
a rigid piece of cardboard or a tray to support it.
Set the matching name card next to each sam-
ple. Conduct a visual inspection. Carefully observe
and sniff each sample.
02
Experiment
Into the Water
It’s a little harder to tell the white substances apart.
In such cases, a chemistry detective first investigates
whether the substance dissolves in water.
You will need: measuring cup, measuring spoon,
pipette, table salt, flour, baking soda, citric acid,
sugar, glucose powder, borax, baking powder,
flavored sugar, washing soda, laundry detergent
Advice for Parents and Adults
Instructions
Warning! — This set contains chemicals
that may be harmful if misused. Read cau-
tions on individual containers carefully. Not
to be used by children except under adult
supervision.
Only for use by children 8 years of age and
older. Use only under careful supervision
of adults who have familiarized themselves
with the kit’s written safety precautions.
Caution! — Contains some chemicals cat-
egorized as hazardous to health. Read the
instructions before use, follow them, and
keep them on hand for reference.
Individual parts may have sharp points, corners,
or edges. Do not injure yourself!
Never bring the chemicals into contact with any
part of your body, especially mouth and eyes.
Keep small children and animals away from the
experiments.
Store the kit out of the reach of small children.
Eye protection for adults not included.
1st Edition © 2010 Franckh-Kosmos Verlags-GmbH & Co. KG, Stuttgart, Germany
This work, including all its parts, is copyright protected. Any use outside the
specific limits of the copyright law is prohibited and punishable by law without the
consent of the publisher. This applies specifically to reproductions, translations and
microfilming and the storage and processing in electronic systems and networks.
We do not guarantee that all material in this work is free from other copyright or
other protection.
Manual and packaging layout: Peschke Grafik-Design, komuniki – Michael Schlegel;
Text: Rainer Köthe, Ruth Schildhauer; Product development: Annette Büchele, Petra
Zimmermann
2nd English Edition © 2013 Thames & Kosmos, LLC, Providence, RI
® Thames & Kosmos is a registered trademark of Thames & Kosmos, LLC.
Translation: David Gamon; Editing: Ted McGuire; Additional Graphics and Layout:
Dan Freitas
Distributed in North America by Thames & Kosmos, LLC. Providence, RI 02903
Phone: 800-587-2872; Email: support@thamesandkosmos.com
Printed in Germany / Imprimé en Allemagne
Contents
Goggles
Pipette
2 Measuring cups, 30 ml
2 Measuring cup lids
2 Test tubes
2 Stoppers for test tubes
2 Measuring spoon (with two
heads)
pH Test strips
Die-cut cardboard sheet
Additional Items
You will need these 16 test substances:
When a scientist finds an unidentified substance,
he or she uses precise examinations and chemical
tests to determine the composition of the unknown
substance. This process is called chemical analysis.
In this kit, you will use simple tools to
investigate a whole range of common household
substances. You will use a few different chemical
investigation methods, along with your eyes and
nose. Of course, you are not allowed to use your
tongue, since some of the materials might be
harmful to your health. Now, let’s get started.
Instructions for Using the Safety
Goggles (Item No. 052279)
Use: The safety goggles are only to
be used with the experiment kit.
Any other type of application is not
permitted. Wear the goggles in such
a way that the eye area is protected.
If necessary, adjust the elastic band
to the child’s head circumference.
The safety goggles can be used with
contact lenses. Wearers of corrective
eyeglasses need special safety goggles
for people who wear glasses.
Duration of Use: Always wear the
safety goggles when performing
your experiments. Not intended for
long-term use. The duration of wear
should not exceed the time of the
experiment.
Storage: Store safety goggles at room
temperature in a dry room. After
the experiment, return them to their
place in the kit box, to keep them
from being scratched.
Cleaning: Do not clean the safety
goggles when they are dry. Rinse
them with clean water and, if neces-
sary, with a mild household liquid
detergent, and dry them with a soft
cloth.
Maintenance: In case of defective
safety goggles or scratched lenses,
exchange them for an equivalently
constructed pair.
Inspection: Check the safety goggles
to make sure they are in good condi-
tion, and replace them if they are
damaged.
Warning: Some extremely sensitive
individuals may experience an allergic
reaction after skin contact with some
materials under some circumstances.
Replacement: These safety goggles
are available as a replacement part.
The safety goggles are tested per EC guideline
89/686/EWG (personal protective equipment)
and EN 166, as well as EC guideline 88/378/EWG
and EN 71-4. Test center per EC guideline 89/686/
EWG and EN 166 Certification Center 0196: DIN
CERTO, Westliche 56, Pforzheim, Germany. Test
center per EC guideline 88/378/EWG and EN 71-4
Certification Center 0197: TÜV Rheinland Product
Safety GmbH, Am Grauen Stein, Köln, Germany
Experiments
• salt
• white flour
• rice
• granulated
sugar
• baking soda
• baking powder
• white laundry
detergent
powder
• coffee powder
• cocoa powder
• finely ground
white pepper
• washing soda
(sodium carbon-
ate, from the
supermarket)
• flavored sugar
such as vanilla
sugar (you can
make your own
by adding a drop
of flavor extract
to a few table-
spoons of sugar)
• tea leaves
(from a tea bag)
• borax (from the
supermarket)
• citric acid (or
instant lemonade
powder
containing
mostly citric acid)
• glucose tablets
(dextrose, from
the drugstore)
You will also need a tea light candle, paper tow-
els, paper plates, and glass jars.
Why?:
Many substances from the kitchen have very differ-
ent appearances. With some white powders, you
can see that they are made of very small crystals,
while you see no crystals in baking soda, glucose
powder, or powdered sugar. Rice comes in little
grains. Flavored sugar, laundry detergent, coffee,
cocoa, and ground pepper have a particularly no-
ticeable aroma. Observe the appearance and aroma
of each substance. That alone will be enough to
identify pepper, tea, coffee, cocoa, and rice.
Place a small measuring spoonful of table salt in
the measuring cup, add two pipettes of warm
water, and stir with the measuring spoon. Wait a
few minutes to see if the salt dissolves. Thoroughly
rinse the measuring cup and repeat the experiment
with each of the other substances. What can you
determine? What does the laundry detergent do?
And what happens to the baking powder when you
add water?
How to Use the Pipette
You will use the dropper pipettes
to add liquids drop by drop.
Squeeze the upper part of the
pipette with your thumb and
index finger and dip the tip of the
pipette into the liquid. As soon as
you release pressure on the bulb,
the liquid will rise up the pipette.
Then, with a light squeeze, you
can add the liquid drop by drop.
After each use, rinse the pipette
thoroughly (fill with water, shake,
and squeeze empty several times).
Why?:
All of the materials here except flour dissolve in
water. In the process, baking powder forms gas
bubbles and laundry detergent forms foam. You can
use this as a simple test to tell flour, baking powder,
and laundry powder apart from the other white
substances.
03
Experiment
Sour or Not?
As you know, vinegar tastes sour — chemists call
it an . Citric acid is also a kind of acid. On the acid
other hand, there are other substances that are, in a
manner of speaking, the counterparts or opposites
of acids. They are called . Chemists are able bases
to determine the level of acidity of a solution (its
so-called pH value) without having to use their
tongues. That’s what the strips of paper are for.
They are called pH indicator strips, and they will
change color to reveal the acidity of a solution.
You will need: 2 measuring cups, pipette,
measuring spoon, pH indicator strips, citric acid,
table salt, granulated sugar, flavored sugar,
washing soda, baking soda, borax, glucose
In one measuring cup, dissolve a
spoonful of citric acid in water. Fil
the other cup with only water.
Cut each pH indicator strip into
four pieces. Briefly dip a piece
into each liquid. Compare the
colors to the pH color scale on
the other side of this instruction
sheet. Repeat the experiment
with each of the seven other
substances listed in the
materials above.
When working
with acids and
bases, it is very
important to wear
safety goggles.
Tip:
Keep the pH indicator strips in their sealed bag
until you are ready to use one, because even the
moisture in the air can alter the indicator strip’s
color slightly. The indicator strip can also stain
your fingers, so wash them thoroughly after
experimentation. Put the used strips on a piece of
scrap paper so they don’t stain your work surface.
Why?:
You can use the test strips to determine the degree
of acidity of a solution, and thereby differentiate
citric acid, borax, and washing soda from the other
white powders.
Test 4
Add one spoon tip of the sample to a measuring
cup and use the pipette to drip some citric acid
solution onto it. Since it doesn’t foam up, this serves
as a safety check. It really can’t be baking soda,
baking powder, or washing soda.
04
Experiment
Gas Factory
Many substances will react chemically with others,
which means that they undergo changes. This can
also be used as an identifying feature. Chemists are
familiar with many such . Citric acid, for reagents
example, can be used as a reagent.
You will need: measuring spoon, die-cut sheet,
large test tube, measuring cup, pipette, pH strips,
citric acid, baking soda, washing soda, baking
powder, borax, table salt, granulated sugar
Assemble the test tube
stand using the parts from
the die-cut cardboard
sheet. Taping the stand to
your work surface makes
it more stable.
Half-fill the large test
ith water, and dissolve ten
ring spoon
acid in it
dly). Label
e test tube
th “citric acid
ution,” insert
e stopper, and
t the solution
de.
05
Experiment
Pretty Crystals
When a substance dissolves in water, it seems to
disappear. In reality, it has just gone into hiding,
and it will reappear when the water evaporates.
Sometimes when that happens, the substance forms
beautiful crystalline shapes, which you can also use
to identify the substance.
You will need: pipette, measuring spoon, 3 old
glass jars, sugar, glucose, table salt
Dissolve four measuring spoonfuls
of sugar in a glass jar with one full
pipette of warm water. Stir until
everything has dissolved. Then
let the jar sit for a few days in a
warm location.
06
Experiment
Heating Things Up
The way a material behaves when heated can also
be an important identifying feature.
You will need: measuring spoon, tea light, plate,
table salt, glucose, sugar, paper towel
Heat some glucose by putting it in the depression
of your measuring spoon and holding it over a tea
light flame. How does it change? What do you
smell? Stop heating Caution — don’t burn yourself!
as soon as the glucose turns brown. Otherwise it
will carbonize and be hard to remove. Finally, rinse
the measuring spoon under running water and dry
it with a paper towel. Next, see how granulated
sugar and table salt behave when heated.
07
Experiment
Test with the Help of the Cards
Using what you learned in the previous
experiments, you are now ready to try an actual
chemical analysis. Let’s start by analyzing a chemical
in a trial situation in which we know the chemical
we are analyzing: sugar.
First write the word “sample” on a piece of paper,
tape it to a clean test tube, and fill it a quarter of
the way with granular sugar. It is on this sample
that you will try out the tests, one after the other in
sequence. The cards from the die-cut sheet will help
you to identify an unknown white substance.
You will need: 11 chemical ID cards, test tube,
measuring spoon, 2 measuring cups, pipette, pH
indicator strips, citric acid solution, sugar, tea
light, plate
Test 1
First check whether a spoon tip of the sample will
dissolve in water. Since it dissolves with ease and
does not produce a foaming or fizzing reaction,
then it cannot be laundry powder, flour, or baking
powder. Eliminate these cards. Save the solution.
08
Experiment
Time to Get Serious
Now try an actual chemical analysis in which you
don’t know the chemical from the beginning. For
example, you could ask your mother or father to
pick one substance from your list, put it into your
test tube “sample” container, and give it to you —
without letting you know what it is, of course.
You will need: chemical ID cards, test tube with
unknown sample, measuring spoon, measuring
cup, pipette, pH indicator strips, citric acid
solution, tea light, plate
Here’s how:
First examine the
unknown substance
with your eyes and
nose, as in Experi-
ment 1. If it’s a white
substance, carry out th
various tests with the
help of the cards as in
the last experiment, un
only one card is left. Th
you’ve done it, and you
yourself a chemistry detective.
Test 2
Test the solution’s pH. Since it is neural, eliminate
the citric acid, washing soda, baking soda, and
borax cards.
Add a spoon tip of baking soda
to a measuring cup, and use the
pipette to add a few drops of the
citric acid solution to it. It will fizz up
vigorously with little gas bubbles. The
the same thing with the other substa
Which ones fizz up and produce gas b
and which don’t? Finally, rinse the pip
other tools thoroughly with water.
Why?:
When combined with citric acid, baking soda and
washing soda create the gas carbon dioxide — the
same gas found in sparkling water. That helps you
differentiate them from other white substances.
Baking powder also exhibits this same foaming
behavior, but water alone is enough to trigger it,
since baking powder is a mixture of baking soda
and a solid acidic substance. Borax does not react
with the citric acid solution.
Glue the stand
together like this.
Experiments
The water will evaporate,
and the sugar will become
visible in the form of
colorless crystals.
Also try the experiment
with the other substances.
Why?:
Not all substances form good
crystals: granulated sugar
and, of course, powdered
sugar form fine spikes,
and table salt forms tiny
cubes. Glucose, on the
other hand, just forms a
whitish glop. You can use
this as a way to differenti-
ate glucose from granulated
sugar (sucrose), which are very
similar in their other properties.
Caution! An adult should be
present for this experiment.
Be sure that nothing catches
on fire, and immediately
extinguish the tea light once
the experiment is over.
Why?:
When heated, sugar decomposes and forms a
brownish caramel, recognizable by its color and its
characteristic smell (just like caramel candy). Table
salt, on the other hand, withstands the heat of
the flame without changing at all. It would need a
much higher degree of heat for a chemical reaction.
You can use this to distinguish sugar from salt.
By now you have gotten to know a few methods
of chemical analysis and the characteristics of some
common substances. Now you will demonstrate
your knowledge by analyzing a substance without
knowing beforehand what it is.
Citric Acid
1.
soluble
2.
red: acidic
3.
4.
no reaction
5.
6.
small angul
Solubility in water
pH test
Visual and olfactory test
no odor
Reaction wit
Crystallizati
Heat test
no reaction
1.0
Laundry Detergent
1.
soluble with foam
3.
smells soapy and clean
Solubility in water
Visual and olfactory test
Flavored Sugar
1.
soluble
2.
yellow-green: neutral
3.
has an identiable aroma
5.
Solubility in water
pH test
Visual and olfactory test
Heat test
browns with caramel smell
4.
no reaction
Reaction with citric acid
6.5
Flour
.
insoluble, settles out
chalky to the touch
Solubility in water
Visual and olfactory test
Glucose/Dextrose
1.
gut soluble
2.
yellow-green: neutral
3.
no odor
4.
no reaction
5.
6.
Solubility in water
pH test
Visual and olfactory test
Reaction with citric acid
Crystallization
no crystals, only white mass
Heat test
browns with caramel smell
6.5
Baking Soda
1.
soluble
s green: basic
or
reaction producing gas
Solubility in water
est
l and olfactory test
on with citric acid
8.5
Granulated Sugar
1.
soluble
2.
yellow-green: neutral
3.
no odor
4.
no reaction
5.
browns with caramel smell
6.
small angular crystals
Solubility in water
pH test
Visual and olfactory test
Reaction with citric acid
Crystallization
Heat test
6.5
Borax
uble
: basic
dor
action
ubility in water
test
al and olfactory test
tion with citric acid
9.5
Table Salt
1.
soluble
2.
yellow-green: neutral
3.
no odor
4.
no reaction
5.
no reaction
6.
small cubic crystals
Solubility in water
pH test
Visual and olfactory test
Reaction with citric acid
Crystallization
Heat test
6.5
4.
strong reaction producing gas
Reaction with citric acid
Baking Powder
1.
soluble with zzing
Solubility in water
Washing Soda
1.
soluble
2.
blue: basic
3.
no odor
4.
reaction producing gas
Solubility in water
pH test
Visual and olfactory test
Reaction with citric acid
11
Test 3
Smell the sample. If it does
not smell like anything,
you can also rule out the
flavored sugar.
Test 5
Now only salt, glucose, and
granulated sugar are left.
Heat a spoon tip of the sam-
ple over the tea light flame.
It turns brown and smells of
caramel. So it’s sugar, and you
can put away the salt card.
Test 6
Let the solution from Test
1 evaporate. This will take
a few days. Small, clear
crystals form. So it can’t
be glucose, which does
not form crystals. Only the
granulated sugar card is
left — and that’s what our
sample is!
Some tests produce no useful reaction, so this infor-
mation has been left off the chemical ID cards.
Why?:
Using this series of tests, you ruled out possibilities
until you determined the identity of the sample
chemical. With these tests (you may not have to
carry out all of them) and the chemical ID cards, you
can identify every substance from the list.
From the color of the pH indica-
tor paper, you can find the pH of
a solution. It ranges from red (a
solution with a pH of 1 is an acid)
to yellow-green (a solution with
a pH of 7 is neutral) to blue (a so-
lution with a pH of 11 is a base).
Tip:
The indicator paper can be
neutralized after use and reused.
Dip the red strips into a basic
solution (for example, the baking
soda solution) and dip the green
and blue strips into an acidic
solution (for example, the citric
acid solution). The paper will
turn yellow again and can again
be used.
pH Color Chart
11
10
9
8
7
6
5
4
3
2
1
Product specificaties
| Merk: | Thames & Kosmos |
| Categorie: | Niet gecategoriseerd |
| Model: | CHEM C100 Test Lab |
Heb je hulp nodig?
Als je hulp nodig hebt met Thames & Kosmos CHEM C100 Test Lab stel dan hieronder een vraag en andere gebruikers zullen je antwoorden
Handleiding Niet gecategoriseerd Thames & Kosmos

5 Augustus 2025

5 Augustus 2025

5 Augustus 2025

5 Augustus 2025

5 Augustus 2025

5 Augustus 2025

5 Augustus 2025

5 Augustus 2025

5 Augustus 2025
Handleiding Niet gecategoriseerd
- Xantech
- Glasdon
- Rollei
- SRS
- Master Lock
- UNGO
- Citronic
- Tanaka
- CkeyiN
- Bliss Outdoors
- Growatt
- Das Keyboard
- Cygnett
- Vacmaster
- Heidemann
Nieuwste handleidingen voor Niet gecategoriseerd

5 Augustus 2025

5 Augustus 2025

5 Augustus 2025
5 Augustus 2025

5 Augustus 2025

5 Augustus 2025

5 Augustus 2025

5 Augustus 2025

5 Augustus 2025

5 Augustus 2025
